Welcome to the second installment of the series on placebo response rate in atopic dermatitis (AD). This series aims to explore the complexities and nuances of placebo response rates in AD clinical trials and dive into the analysis of high response rates. This article explores the concept of a reasonable placebo-response rate in atopic dermatitis (AD) clinical trials. It defines influential factors, such as disease severity, experience, monitoring, and the use of photography. The article also discusses the challenges and considerations in setting realistic expectations and ensuring high-quality efficacy evaluations.
What is considered as a reasonable placebo-response rate?
Let’s consider what constitutes a reasonable response in an AD study conducted in patients with moderate to severe AD, where the primary endpoint is change from the baseline in EASI.
Is a 60% change reasonable? Many would argue that this is exceptionally high and problematic, as most studies do not observe a 60% change from baseline overtime.
Is a 0% change reasonable? Most would argue that it is not reasonable given that patients experiencing flare-ups followed by spontaneous improvements at baseline and screening and that some patients will improve because of higher adherence to moisturizer used. A 0% placebo response rate may suggest that there were issues with evaluations.
In fact, as an investigator, I cannot recall any large AD study with a 0% placebo response. Therefore, a reasonable response likely lies somewhere in between these extremes.
The goal is to conduct a trial that will generate accurate safety and efficacy data about the product being studied. A 60% placebo response is likely not ideal as it leaves very little room to demonstrate a difference between the active treatment and the placebo. Similarly, a 0% change is probably not ideal either, as if the same situation applies to the active treatment, the measured effect size will be less than the actual effect.
Disease Severity
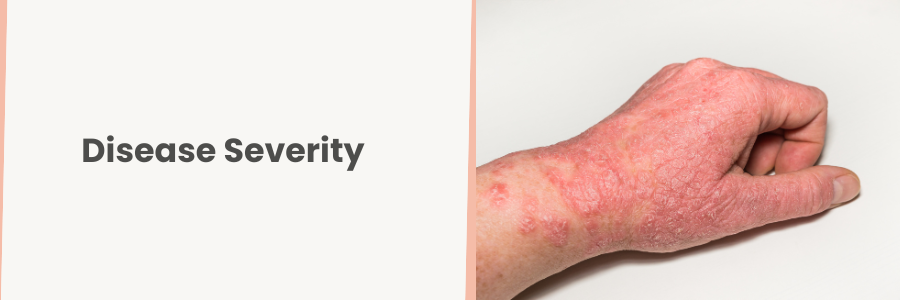
It is important to set realistic expectations. As previously mentioned, patients with mild disease, for example, 1% BSA with mild IGA, treated with a topical drug, will have a significantly higher placebo response rate that those in a study with moderate to severe BSA 10, EASI 16+ using a systemic medication.
Experience

It is important to involve sites with a wealth of experience in conducting AD studies and that are accustomed to performing clinical evaluations of severity. It is beneficial to include sites with a proven track record of high-quality efficacy evaluations. CROs and sponsors frequently active in dermatology are likely to be aware of which sites consistently deliver high-quality results.
Monitoring

CROs and sponsors should consistently track changes in efficacy evaluations throughout the study to pinpoint outlier sites or sites where, as previously mentioned, the evaluations appear illogical.
Photographs

Photography can also be a useful tool. Despite the challenges of photographing AD due to its nature, requesting sites to capture images of the most severe areas can be beneficial. If, upon review, the photos from a study requiring moderate to severe patients consistently suggest a severity of almost clear or mild, then there is likely a problem that needs to be addressed.
Conclusion
To conclude, managing placebo response rates in AD clinical trials involves balancing rates between 0% and 60%, setting realistic expectations based on disease severity, and choosing experienced study sites. Consistent monitoring and the use of photography can aid in assessing AD severity.