Partner with Our Biometrics Team
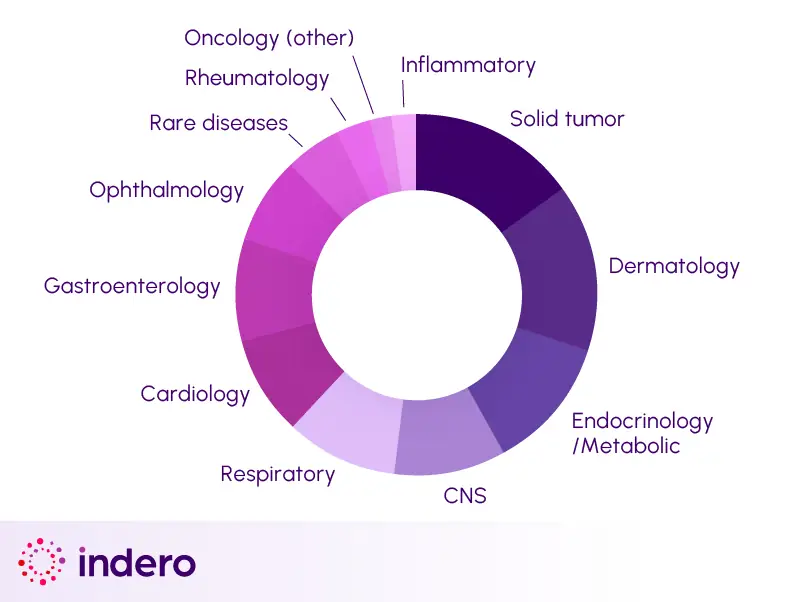
For 25 years, our biometrics services have consistently been exceeding sponsor expectations with reliability, quality, and expertise. We handle complex study designs across all trial phases, utilizing innovative data analysis techniques and methodologies. Share your data management and biostatistics needs with us today.