Palmoplantar Pustulosis (PPP) Case Study
Click here for a PDF version of this case study

Outcomes
Innovaderm successfully managed this PPP study
and this resulted in:
Enrollment completed 3 weeks ahead of schedule;
Successful study and satisfied sponsor
Study Characteristics
-
- Study Phase: II
- Patient Population: Palmoplantar pustulosis
- IP Route of Administration: Systemic
- Sites Distribution: 17 sites in 2 countries (Canada, Germany)
- Study Complexities: Presence of active lesions required, high PPPASI and PPPGA
Study Challenges and Solutions
Challenges
Solutions
Site Selection
- Identification of sites due to limited pool of patients with rare diseases
-
- Early activation of sites in Germany
- Timely assessment of potential challenges with site selection
- Implementation of in-house central campaign
Outcome: Enrollment completed 3 weeks ahead of schedule
Recruitment
- Participant apprehension regarding biopsy consent
- Hassle of transporting IP between site and patient's home
- Short treatment period deterred interest in participation
-
- Strong presence of on-site staff to address questions or concerns related to participation
Outcome: Successful study and satisfied sponsor (+40%)
Key Success Factors & Strategies
Activation of sites in Germany to bolster recruitment
Effective in-house central campaigns
Project team’s tremendous engagement with sites to drive recruitment
Recruitment Curve and Site Activation
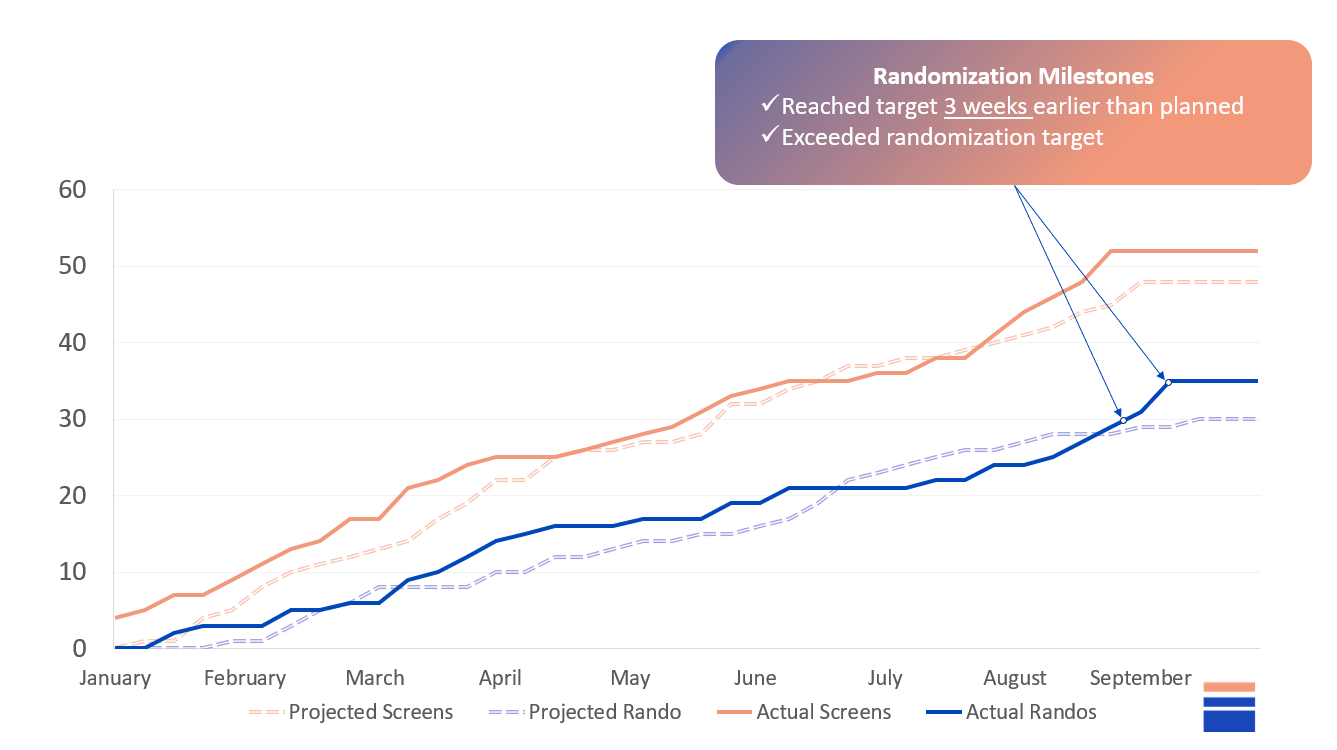
Enrollment was completed 3 weeks earlier than planned
Newsletter
Newsletter subscription resources

