Hidradenitis Suppurativa (HS) Case study
Click here for a PDF version of this case study

Outcomes
Innovaderm successfully managed this HS study
and this resulted in:
Enrollment completed 3 months ahead of schedule
Study Characteristics
-
- Study Phase: II
- Patient Population: Hidradenitis Suppurativa
- IP Route of Administration: Systemic - Subcutaneous
- Site distribution: 25 in North America and 16 in Europe
Study Challenges and Solutions
|
|
Challenges |
Solutions |
||
|
|
Recruitment |
Outcome: Screenfailure rate reduced due to early identification of lab errors and lab patients’ tests adequately screened |
||
|
|
Shipments |
Outcome: Significant reduction in shipping costs |
||
|
|
Academic sites |
Outcome: Early-stage discussions with PIs helped streamlined recruitment and start-up timelines |
Key Success Factors & Strategies
Optimal site selection: experienced PIs in HS and experienced Innovaderm sites
Implemented broad reach digital ad campaigns / assisted sites with the development of recruitment material
Recruitment Curve
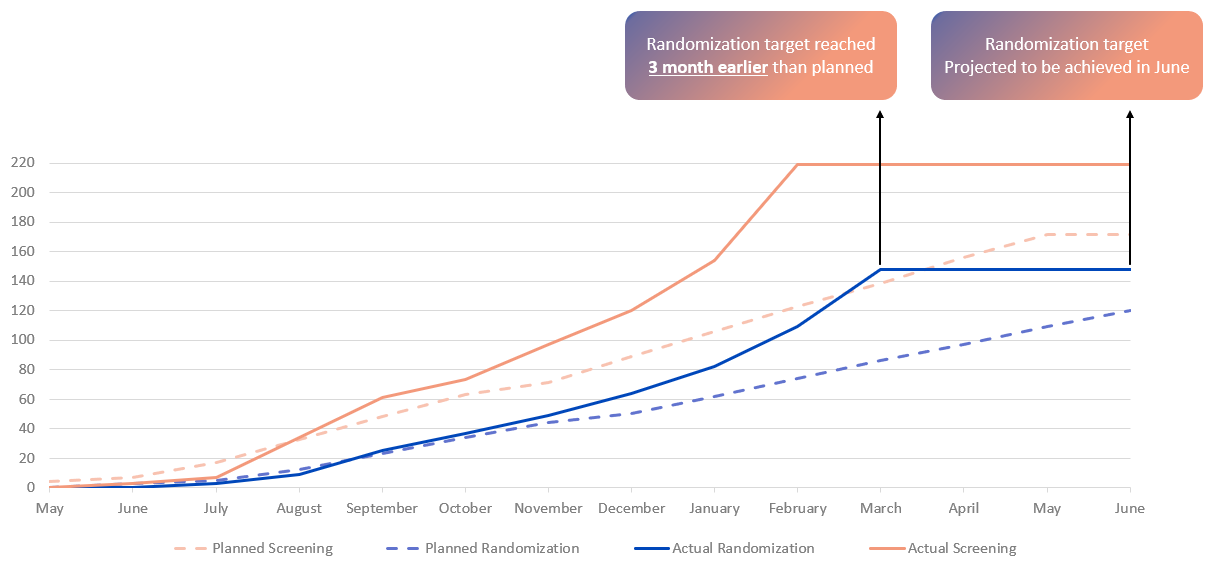
Enrollment completed 3 months ahead of schedule
Newsletter
Newsletter subscription resources

