How to Write First-Rate Protocols for a Psoriasis Study - Focus: Study Design

The success of any clinical study is dependent upon a study protocol; the overall quality of the study depends on this very document. This is especially true for multicenter studies which involve many investigators and clinical staff. This article will explore the best tips to deliver first-rate protocols in psoriasis to set you apart from the competition. Innovaderm Research Inc specializes in a spectrum of dermatological indications, namely acne vulgaris, atopic dermatitis, and psoriasis.
What is plaque psoriasis?
Plaque psoriasis, characterized by thick, dry scales, is a chronic, relapsing, inflammatory type of skin disease with a high prevalence worldwide of 2 to 3%. It is often associated with pruritus, and with comorbidities such as psoriatic arthritis and obesity. The disorder tends to peak among individuals between 15 and 20 years of age and 55 and 60 years of age.
Common triggers for plaque psoriasis include certain medications, infections, injuries to the skin, and stress.
Insights into study design
Prior to drafting a protocol for psoriasis, it is crucial to assess the competitive landscape related to the indication under investigation and to identify the current clinical development stage of the investigational study drug.
In a Phase 1 study, the safety and pharmacokinetics (PK) of the study drug are evaluated through intensive lab testing, electrocardiograms (ECGs), and vital sign assessments to provide full safety and PK profiles. Biomarker assessments can also offer insight on efficacy in small early phase trials.
In proof-of-concept (POC) and Phase 2 studies, the choice of efficacy endpoint should prioritize the one exhibiting the highest sensitivity or greatest capacity to discern disparities among treatment groups. In Phase 3 studies, the protocol should be aligned as closely as feasible with the requirements of regulatory agencies.
Types of study design
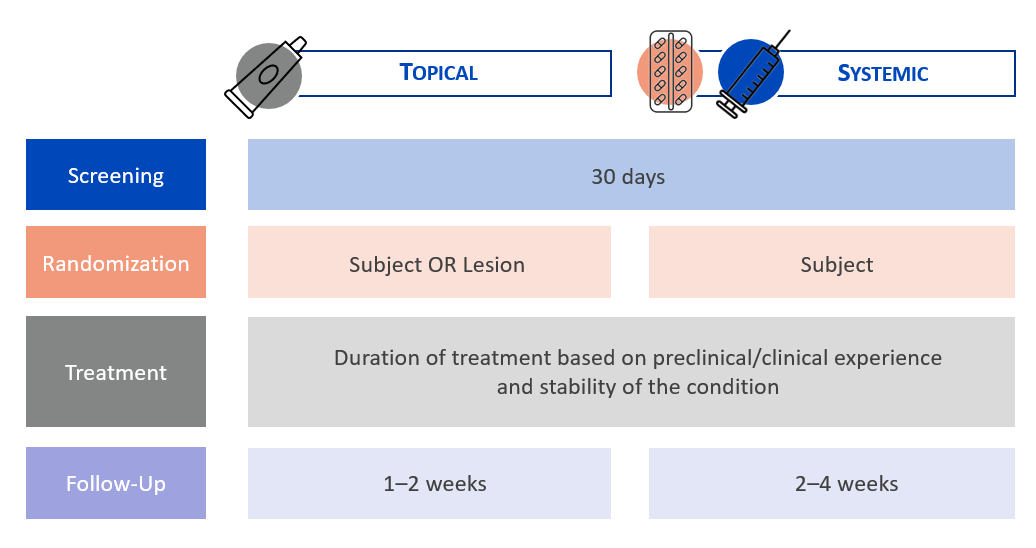
When designing a clinical study on plaque psoriasis, it is imperative to consider the distinctive features of the disease pathology. For instance, the design protocol for plaque psoriasis would significantly differ from one intended for palmoplantar pustulosis, which represents the most prevalent manifestation of pustular psoriasis.
A screening period of 30 days is recommended for psoriasis studies involving topical or systemic agents. This allows subjects who are taking medications that need to be stopped at least four weeks (28 days) prior to baseline to do so within the screening period and therefore reduce the screen failure rate compared to a screening period of 28 days.
In contrast to atopic dermatitis, plaque psoriasis is a very stable condition. Consequently, it is feasible to conduct POC studies at a limited number of study centers, involving a small cohort of subjects with a low body surface area (BSA) involved with psoriasis.
In clinical trials for psoriasis utilizing topical products, randomization can occur either at the subject level or be within subjects, which is referred to as an intraindividual study. An intraindividual study entails the application of various study products (potentially featuring different concentrations) to several target areas on an individual’s body. Employing an intraindividual design offers several advantages, including the mitigation of inter-subject variability and decreased necessity for a large number of subjects to achieve statistically significant results.
Treatment duration in psoriasis trials
Developing a first-rate protocol also entails establishing the drug dosage treatment duration. It is noteworthy that the erythema resulting from skin lesions in psoriasis can span several months. Consequently, a longer treatment duration with a primary endpoint at Week 16 should be favored in a POC study, especially when evaluating study drugs with a new mechanism of action.
In Phase 2/3 studies, the recommended treatment duration is 12 to 16 weeks. This should be respected not only when designing a clinical study on psoriasis but also upstream during the toxicology evaluation. Toxicology is too often assessed in a short period when it should, in fact, last about 16 weeks.
Key eligibility criteria
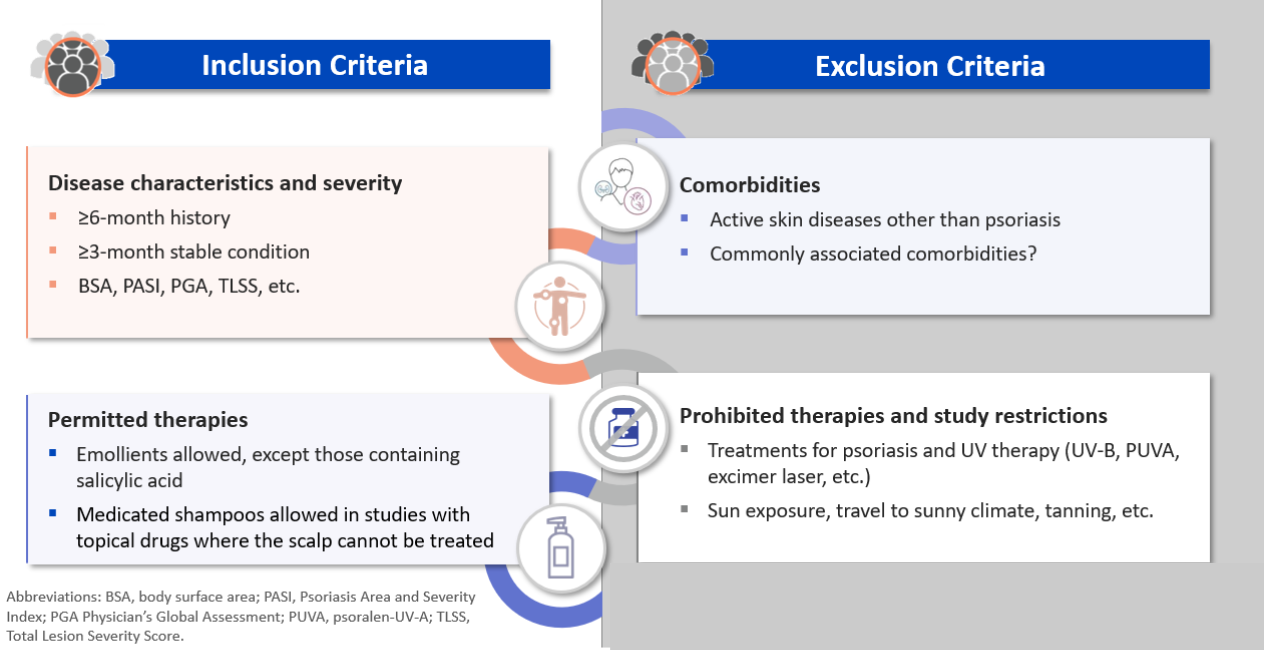
The next step in protocol writing is to determine the population which will be enrolled in the study.
Eligibility is based on a person’s medical history and the stability and degree of severity of their condition using clinical assessments such as the Physician Global Assessment (PGA), the Psoriasis Area Severity Index (PASI), etc.
When determining exclusion criteria in psoriasis, comorbidities tend to be a key area of focus. Active skin diseases, such as predominantly pustular psoriasis, should be excluded. However, criteria related to commonly associated comorbidities such as obesity, for example, should never be too rigid in order to avoid hindering subject enrollment and have a representative population.
In addition, it is essential to clearly delineate which therapies are permitted and prohibited. Emollients and baths/showers have a limited impact on psoriasis and should therefore be permitted. In studies involving topical drugs, where the scalp cannot be treated despite it being one of the primary active areas of psoriasis, medicated shampoos should also be permitted.
Regarding restrictions, sun exposure can greatly affect psoriasis. Any subject planning a trip to a warm-weather destination during the study should be excluded.
Exploring the role of study endpoints
Contingent upon the study phase and study treatment, the primary efficacy endpoint can be based on the target lesion severity score (TLSS) for early phase studies on topical treatments, the PASI, or the PGA.
In studies including subjects with a low BSA, PASI may lack the necessary sensitivity, and it is advisable to consider using the PGA or PGA x BSA as the primary endpoint. Additionally, the study should encompass assessments of pruritus and evaluations related to quality of life.
Quality of Life (QoL) tools should be considered in all phases of development as they can offer many advantages depending on the sponsor’s needs. In psoriasis studies, the Psoriasis Symptoms and Signs Diary, is another frequently used questionnaire, and a validated tool accepted by the FDA and used for label claims.
Conclusion
A psoriasis protocol must portray a solid understanding of the indication and what is being measured. Be it procedures for a study on psoriasis or another skin indication, the best protocols in dermatology are often the simplest, with the goal of bridging a knowledge gap or highlighting a novel concept in the field. Stemming from a strong collaborative effort, a first-rate protocol provides a roadmap for better enrollment rates and data quality while allowing investigators to remain at the forefront of dermatology research.
For more great tips, watch our webinar on the Art of Protocol Writing.
***
Want to ensure your study is a great success? Contact us today to partner with our expert team of clinical trial professionals!
About the Author
Julie Basque is a senior clinical scientist at Innovaderm with more than 15 years’ experience in clinical research within a CRO environment. Throughout her career, she has achieved proficiency in scientific and regulatory affairs, protocol development, study design, and study report writing. Julie is recognized for her attention to detail, critical thinking, and mentorship.
Stay updated on the latest in the dermatology industry by subscribing to our newsletter. Do not miss out on valuable insights and research updates. Join our scientific community today
Newsletter
Newsletter subscription resources

