Atopic Dermatitis (AD) Case Study
Click here for a PDF version of this case study

Outcomes
Innovaderm successfully managed this AD study
and this resulted in:
Enrollment completed 2 months earlier than planned;
Innovaderm CRU recruited 33% of patients
Study Characteristics
-
- Study Phase: Ib
- Patient Population: 36 subjects with moderate to severe AD
- IP Route of Administration: Systemic - Oral
- Site distribution: 10 sites in 2 countries (USA and Canada)
- Study Design: Randomized, sequential, placebo-controlled, double-blind | Multiple ascending dose (MAD): 3 sequential cohorts
- Study procedures: 4.5 mm punch skin biopsies, photographs, biomarker analysis, skin microbiome, pharmacokinetics, tape stripping and efficacy-safety assessments
Study Challenges
-
- Recruitment during summer months; Seasonality impacts on AD flares
- Short treatment duration; 4-week treatment period
- Data and Safety Monitoring Board (DSMB) after each cohort; Challenging to manage without affecting recruitment
Key Success Factors & Strategies
Recruitment completed in 6 months vs planned 8 months
Outstanding contribution by Innovaderm's Clinical Research Unit
Recruitment Curve
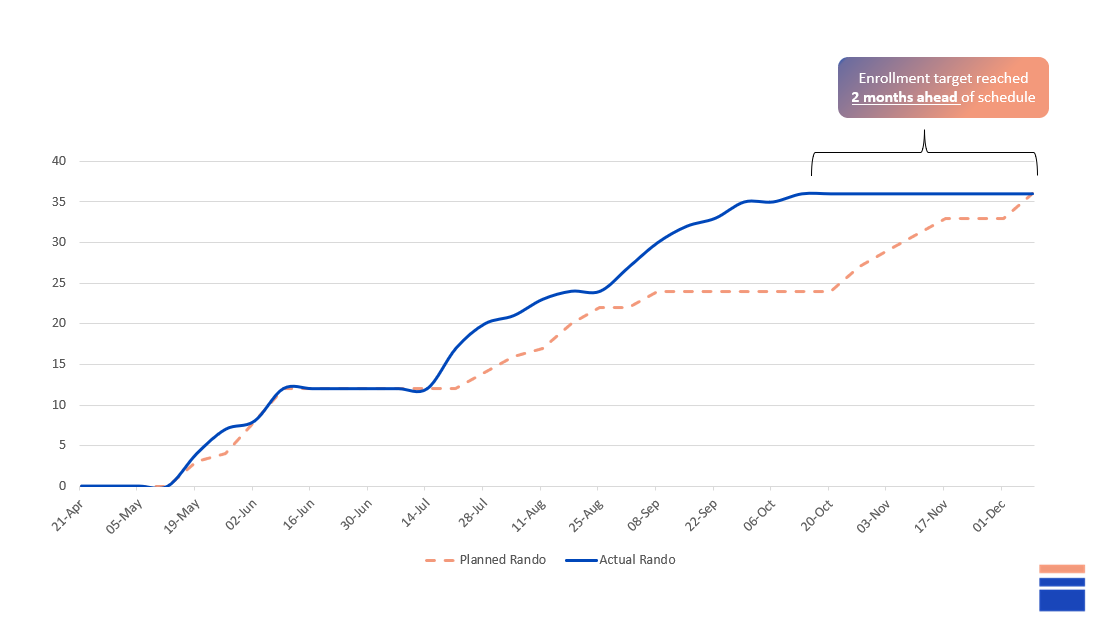
Enrollment target reached 2 months ahead of schedule
Newsletter
Newsletter subscription resources

