Using Biomarkers in Clinical Research
| Table of Contents |
| 1. Why use Biomarkers in Dermatology Clinical Research? |
| 2. Sensitivity of Biomarkers Analysis |
| 3. Limitations of Biopsies in Biomarkers Studies |
Clinical biomarkers ushered in a new era of possibilities in medicine by profoundly impacting the way we investigate and develop new treatment options. Non-invasive techniques such as tape stripping and transdermal analysis patch (TAP) are transforming the dermatology clinical landscape. Biomarkers in clinical trials measure changes in protein, gene expression, or other biochemical pathways associated with a specific condition or response to a drug.
Unlike subjective visual assessments performed by clinical investigators, biomarkers are often more sensitive and accurate in detecting efficacy, thus providing a more comprehensive picture of the effects of treatments.
Why use Biomarkers in Dermatology Clinical Research?
Using biomarkers in clinical trials offers several advantages. The 4 main reasons for using biomarkers:
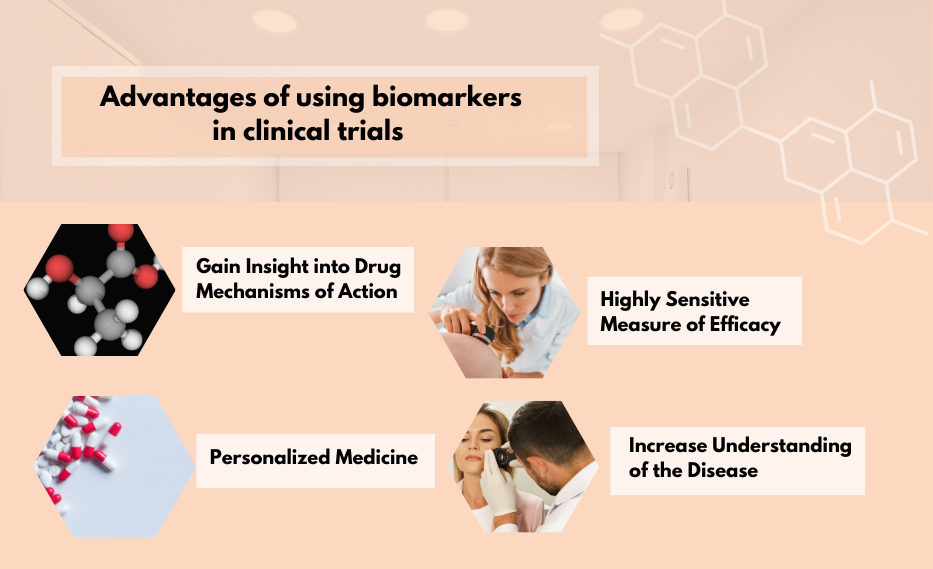
1. Gain Insight into Drug Mechanisms of Action
Biomarker studies provide an effective way of understanding how drugs work. They can provide a comprehensive assessment that can be done very early in the process of drug development.
2. Highly Sensitive Measure of Efficacy
Biomarkers are often more sensitive than the human eye which is one of the most common methods of efficacy measurement for inflammatory skin diseases, rendering them ideal for clinical trial use.
3. Personalized Medicine
This has unfortunately not yet reached inflammatory skin diseases and yet integrated in daily use by dermatologists. However, this could change in the coming years. Indications where drugs are very effective and well tolerated are probably not ideal for the use of biomarkers. However, for many inflammatory diseases, our current treatments usually only induce partial responses. Biomarkers that could predict responder patients, or patients more at risk of severe adverse drug reactions, would be useful.
4. Increase Understanding of the Disease
Biomarkers can provide valuable information about the underlying cause of a disease and can be used to identify targets for further research. This could lead to the development of new treatments or drugs that may better address the patient’s needs.
Sensitivity of Biomarkers Analysis
Case 1: atopic dermatitis study
A phase 1 study in atopic dermatitis, published many years ago in the New England Journal of Medicine, compared different doses of dupilumab to placebo. Biopsies and clinical evaluations were done at baseline and week 4. Based on typical evaluations performed in atopic dermatitis research, results showed that the endpoint on EASI-50 was met at week 4 with the 300 mg and the 150 mg doses of dupilumab (p-values between 0.05 and 0.003). The same results were observed for the pruritus NRS, with p-values between 0.01 and 0.05.
However, when the transcriptome was analyzed by Dr. Guttman’s laboratory, there was a statistically significant difference for both the 150 mg and the 300 mg compared to placebo, but the p-values were significantly smaller.
Case 2: atopic dermatitis study
In a subsequent study conducted at Innovaderm a few years ago, the results from the visual assessments performed by clinical investigators were compared to biomarker results from skin biopsies. Optimal clinical conditions were put in place to facilitate the comparison between the 2 types of evaluations. As such, this study was conducted at a single clinical research unit located in Montreal (the Innovaderm CRU) by dermatologists who are knowledgeable with atopic dermatitis clinical evaluations. This study used the mini-zone type of approach where all topical products were concomitantly applied on small areas of skin in each patient to facilitate the comparison between the areas treated with the drugs and the adjacent non-treated area. This is probably the most sensible way of seeing clinical differences.
For this study, 30 patients with atopic dermatitis were recruited and 4 products (clobetasol propionate, betamethasone dipropionate, pimecrolimus and vehicle) were applied to different small areas of the skin of each patient. The first clinical evaluation (i.e., Total Skin Score (TSS)) showed a statistically significant difference between the 2 steroids and the vehicle. However, at the end of the 15-day study, there was no significant difference between pimecrolimus and vehicle, although the change from baseline with pimecrolimus was numerically higher than the vehicle.
Similar results were observed with biomarkers: the steroids performing significantly better than vehicle in terms of IL-13 and IL-22 in skin biopsies, and pimecrolimus performing numerically better than vehicle, but with no statistically significant difference.
By using conditions that are ideal in terms of detecting efficacy with the human eye, the results were determined equivalent to biomarkers. According to Dr. Robert Bissonnette’s professional experience, it was observed in the last 5 years in several instances that biomarkers were more sensitive than the clinical evaluations.
Limitations of Biopsies in Biomarkers Studies
It is a known fact that biopsies can be a challenge in clinical trials. Some elements to keep in mind are:
1. The negative impact on recruitment
This aspect can vary depending on the targeted population and the location of biopsy. Younger patients tend to be less interested in skin biopsies than older patients. A biopsy on the lower back for a psoriasis study is very different from a biopsy on the face in an acne study, for example.
2. Withdrawal of consent for second biopsy
Another aspect to keep in mind is that regardless of the number of patients that consent to biopsy collection in a study, it has been recorded that some patients tend to refuse a second biopsy. From the CRO standpoint, we have experienced such issues in certain trials, particularly those involving younger patients. When a significant number of participants decline the second biopsy, it can impact the primary endpoint and the study outcome. This situation has been recorded mainly with a younger population of patients where they tend not to heal as well as older patients and they tend to be more concerned about the aesthetic outcome of the biopsy especially at week 4 or week 12 since the biopsy sites are very red at that point in time.
3. Sensitive target areas
The skin area has a big impact on the recruitment specifically for areas where biopsies are hard to perform, such as palms of the hands and soles of the feet. Biopsies in those areas are painful and the proportion of patients who get infections is higher, which increases the number of patients who will refuse a second biopsy. Pain in general can also be a deterrent to accept a skin biopsy, particularly in children.
4. Limit in the number of biopsies
From an ethical point of view, there is a limited number of biopsies that can be collected per study as well as a limit of biopsies performed per day. These limitations will have to be considered in the study design.
Considering the constraints related to biopsies listed above, non-invasive methods could be considered as an effective alternative. To learn more about non-invasive methods to analyze biomarkers, follow the links to Innovaderm’s articles on tape stripping, and transdermal analysis patch (TAP).
Biomarkers offer a unique opportunity to assess the efficacy of treatments in clinical trials with often a greater accuracy and sensitivity than traditional visual assessments. The implications of biomarkers on future trials are promising, and further research on their use should be conducted to understand how they can be leveraged. Ultimately, biomarkers have the potential to revolutionize clinical trial design and improve patient outcomes in the years ahead.
About the Author:
Julie’s experience exceeds 10 years in the domains of clinical pharmacology, medical writing, and scientific affairs. This extensive professional journey has been predominantly within the dynamic CRO environment. Julie assumes a pivotal role as medical writer within Innovaderm’s Scientific Affairs Department.
Let’s shape the future of research and make a difference in the industry, gain Innovaderm’s support in your upcoming trial and propel your study to new heights.
Newsletter
Newsletter subscription resources

