Palmoplantar Pustulosis (PPP) Rescue Case Study
Click here for a PDF version of this case study

Outcomes
Innovaderm successfully transitioned this rescue study from another CRO and this resulted in:
Increase in enrollment of over 10x
Tripled site performance post transition
On-time patient recruitment
Study Characteristics
- Study Phase: II
- Study Status: Rescue study
- Patient Population: Palmoplantar pustulosis (PPP)
- IP Route of Administration: Systemic
- Original Sites at Rescue: 20 in 2 countries (USA, CAN)
- Post Rescue Final Sites Distribution: 40 in 4 countries (US, CAN, GER and POL)
Study Challenges
Only 3/20 legacy sites had recruited subjects over a period of almost one year
-
- Only 8% of subjects enrolled when Innovaderm took over study in Dec 2019
- Legacy sites did not have target patient population in current clinical practice
- Low motivation at legacy sites when Innovaderm took over
- COVID 19 restrictions impacting sites as of March 2020
Key Success Factors & Strategies
Country and site selection
PI training
Site activation
Site engagement
Study awareness
Recruitment Curve and Site Activation
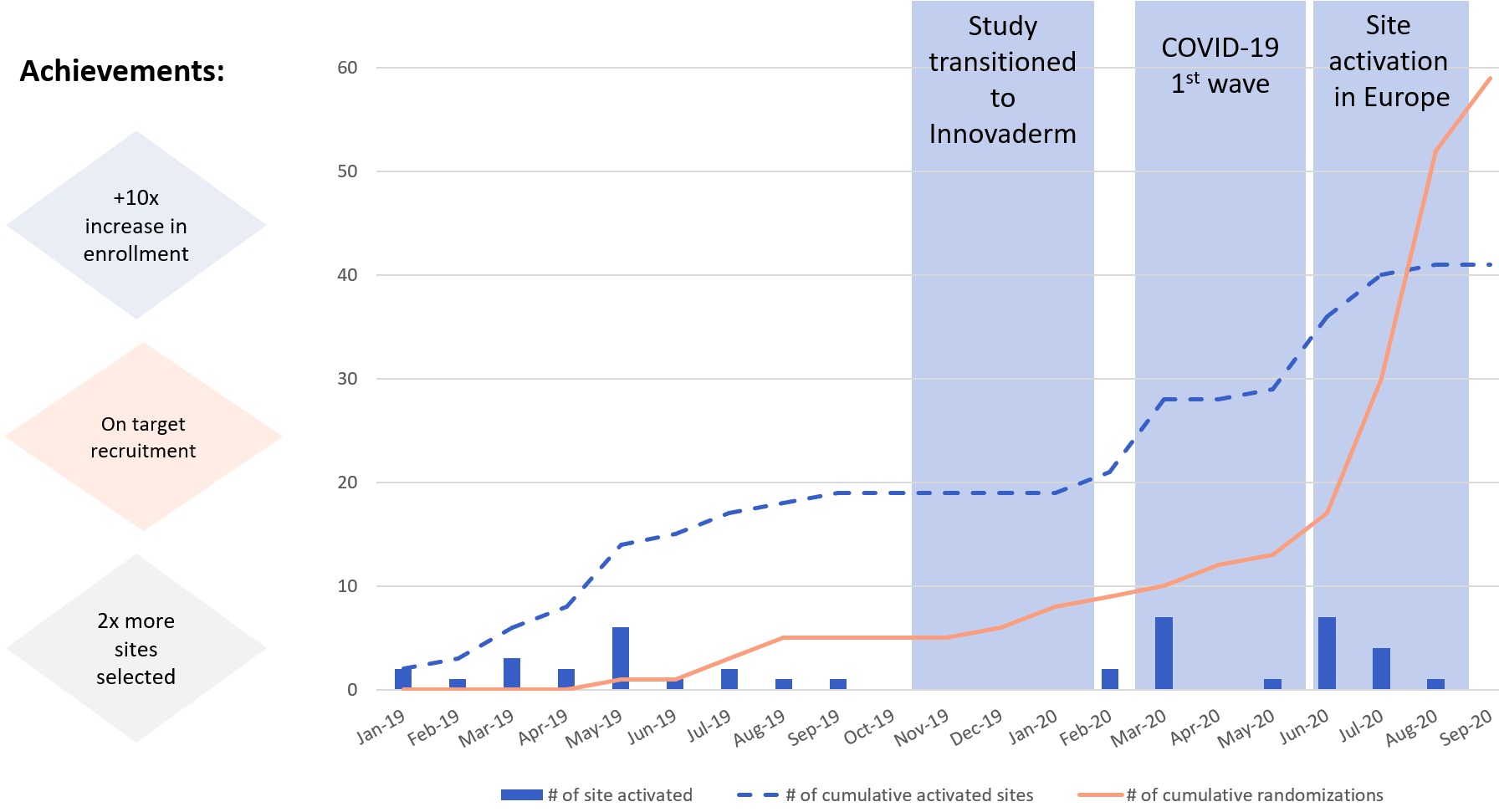
Site Performance before and after transitioning to Innovaderm
| Site / Status | Before Transition (pt/site) | After Transition (pt/site) |
| Innovaderm’s sites | N/A | 2.16 |
| Previous CRO sites | 0.58 | 1.80 |
Performance of sites from other CRO tripled after transitioning to Innovaderm
Innovaderm had the most performant sites
Our Key to Success
-
- To identify the top sites in the right locations (i.e., country)
- Years of experience with dermatology sites
- To focus on the sites most likely to make a difference in recruitment and assure quality data
- To focus on the sites most likely to make a difference in recruitment and assure quality data
- Site Engagement
- Use the momentum of activation to quickly bring FPI to each site
- CRAs in close contact with site throughout the study
- To identify the top sites in the right locations (i.e., country)
Newsletter
Newsletter subscription resources

