Enhancing Clinical Performance and Cost Efficiency: The Significance of Protocol Writing in Dermatology Trials

| Table of contents |
| Best tips to deliver first-rate protocols |
| 1. Poor Quality vs. High Quality Protocols |
| 2. Impact of Protocol Amendments |
| 3. Key Considerations |
| 4. Advantages of a Protocol Template |
Did you know that protocol amendments are not only common, but also very costly? In fact, as per a recent article by Getz K. et al., 57% of protocols had at least one substantial amendment with an average of 2.3 global amendments for phase 2 and phase 3 studies. The cost to implement one protocol amendment was $141,000 US for a phase 2 study and $535,000 US for a phase 3 study. To avoid unnecessary hassle and expenses, take the time to ensure your protocols are well written and top-notch quality.
The success of any clinical study is dependent upon a sturdy protocol; the overall quality of the study depends on this very important document. This is especially true for multicenter studies which involve many investigators and clinical staff. This article will explore the best tips to deliver first-rate protocols in dermatology to set you apart from the competition. Innovaderm Research Inc specializes in a spectrum of dermatological indications, namely acne vulgaris, atopic dermatitis, and psoriasis.
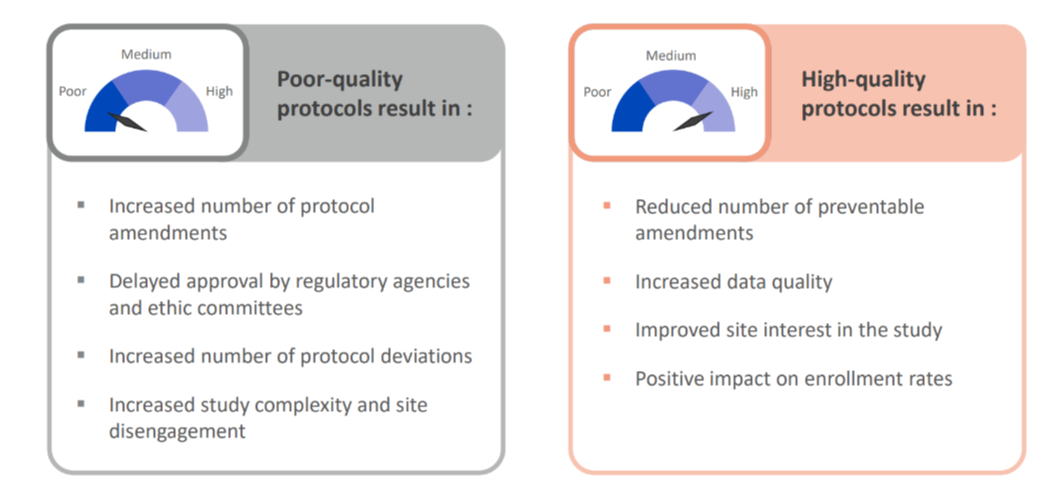
Poor Quality vs. High Quality Protocols
The clinical trial protocol is considered the foundation of the clinical trial. In the startup phase of the study, the focus is often on timelines which can increase the pressure on the finalization of the protocol. While finalizing the protocol is an important milestone in the study startup phase, making sure it meets high quality standard is essential to ensure study success.
A poorly written clinical trial protocol in dermatology may result in an increased number of protocol amendments and consequently greatly impact timelines, costs, and delays in the approval by regulatory agencies and ethics committees. A poorly written protocol can also increase the number of protocol deviations, thereby compromising data quality. Additionally, a poorly designed protocol may increase the level of complexity of a given study which ultimately can lead to site disengagement.
High-quality protocols tend to have a reduced number of preventable amendments, an increase in data quality, site interest in the study and ultimately, a positive impact in enrollment rate and overall study success.
Impact of Protocol Amendments
Writing a high-quality clinical trial protocol from the start reduces the number of preventable amendments. According to Getz K. et al., it has been shown that more than half of protocols had at least one substantial amendment. Among these amendments, half were preventable.
Another interesting fact is that protocols with at least one substantial amendment had a reduced enrollment rate compared to the protocols with no amendments. Protocol amendments can greatly influence clinical trial performance and cost. It is therefore critical that quality standards are met during protocol development to avoid the burden and delays caused by revisions.
Key Considerations
Advantages of having a clinical scientist as the protocol author
One of the first steps prior to protocol development is to ensure that the team has experience and in-depth knowledge of the therapeutic area. Clinical scientists typically have a strong background in research, which is a great asset in the early phase of study design.
Throughout the course of a study, the clinical scientist is also involved in the preparation of various clinical documents, including study reports and publications. They provide scientific expertise to the operational team, which results in a better understanding of the challenges that can occur during a study, as well as considerations for future protocols.
Oversee the protocol review process
A CRO should have established procedures in place for the development and review of a research protocol in order to deliver a comprehensive document that clearly defines the purpose of the study. To achieve this, the clinical scientist collaborates with many experts throughout the process including a project manager, data manager, biostatistician, clinical site representative (ex. study coordinator and investigator), and a specialist in Regulatory Affairs.
Use a patient-centric approach
The participation of an internal multi-disciplinary team from a specialized CRO ensures an efficient review process and that every aspect of the study is adequately described in the protocol. Getting a clinical trial protocol reviewed by study coordinators and investigators provides great insight from an execution perspective. Working closely with the clinical site staff who are in direct contact with patients optimizes the protocol, it allows consideration of the patient’s perspective, and increases site engagement for the study.
Develop your KOL network
Additionally, cultivating high-quality relationships with key opinion leaders (KOLs) in the therapeutic area of interest is also significant for the development of a robust study protocol.
A collaborative approach involving the different teams reduces the chance of error and the need for future amendments. It also allows the identification of risks early in the study and the time to plan mitigation measures accordingly.
The mentioned considerations reinforce the importance of working with a CRO’s internal team starting at the protocol writing stage.
Advantages of a Protocol Template
It is imperative that the writing capabilities of your CRO be assessed prior to the development of any protocol as well as an established review process is in place.
The use of a protocol template is common in the clinical research industry. It should be adapted to the area of expertise to provide support to the clinical scientist during the writing phase.
A good template should feature design considerations specific to different indications in dermatology and include handy tips based on previous studies and experience. To ensure efficient and rapid writing, a template should have a large library of the most common efficacy evaluations and Patient-Reported Outcome (PRO) descriptions, which are aligned with the current literature.
In addition, a protocol template will provide a standard structure that facilitates the review by the internal teams and helps streamline the process for improved timelines.
Finally, the use of checklists is essential to verify that key elements are included in the protocol, reduce the risk of errors, and need for future amendments.
Conclusion
A first-rate clinical trial dermatology protocol requires the following elements: a solid understanding of the therapeutic area, a comprehension of the study objective, and alignment with current literature in the field. Stemming from a strong collaborative effort, a first-rate protocol not only provides a roadmap for better enrollment rates and data quality, but also allows investigators to remain at the forefront of dermatology research.
For more great tips, watch our webinar on the Art of Protocol Writing.
***
Want to ensure your study is a great success? Contact us today to partner with our expert team of clinical trial professionals.
About the Author
Audrey Fortier is an Associate Director, Scientific Affairs at Innovaderm. She is recognized for her expertise in therapeutic and medical writing and excellent mentorship. She has a remarkable 15 years of experience in the CRO space and 7 of which she dedicated to Innovaderm Research. She exemplifies excellence in every aspect of her role. Her professional growth parallels our organization's development and expansion.
Newsletter
Newsletter subscription resources

