Navigating AI risks in healthcare just got easier with the FDA's new draft guidance and explanations from Guillaume Gigon, Innovaderm's VP of Technology and AI. You can even comment on the FDA draft document by April 7. Interested in learning more? Contact us Newsletter Join our science community with Press Forward, our quarterly newsletter. […]

In the ever-evolving landscape of clinical research, Phase I studies are becoming increasingly complex. We’re proud to announce that two of our trailblazing colleagues, Ana Palijan, Director, Early Phase and Translational Research, and Éric Hardy, Senior Director, Biometrics, have contributed to a groundbreaking white paper that explores these complexities. Their expert analysis highlights the challenges […]

Contract Research Organizations (CRO) are the backbone of the clinical research industry, driving research and development that leads to new therapies and treatments. With a multitude of CROs in the market, each offering a unique set of services and expertise, identifying the frontrunners in dermatology CRO arena can be a daunting task. This challenge is […]

Table of Contents Corrective Data Mining Best Practices to Accelerate Recruitment and Local Advertising Consider Using Imagery in Pre-Screening Digital Advertisement Campaigns and Their Impact Secondary Screening Recruitment Start Date In the ever-evolving landscape of clinical trials, patient recruitment strategies stand as a critical component. The ability to effectively and efficiently recruit patients can significantly […]

Project Overview The client sought support for their pre-IND (Investigational New Drug) submission and development program. Our goal was to contribute to the clinical section of the pre-IND submission package and prepare clinical questions for their Vitiligo asset. Steps and Actions Taken Steps Actions Pre-IND Submission Development Clinical Section […]

Table of Contents Common Types of Backup Plans in Rheumatology Clinical Trials Adapting to Evolving Therapies in Rheumatology Trials Backup Countries Protocol Amendments: A Proactive Strategy Positive Impact of Backup Sites on the Conduct of the Study Financial Impact of Backup Sites Rheumatology clinical trials, by their very nature, are unpredictable. Unforeseen complications can arise, […]

The American College of Rheumatology (ACR) Convergence 2024 in Washington, DC, has concluded, leaving the global rheumatology community with a wealth of insights and innovations. As one of the largest rheumatology conferences worldwide, it brought together experts in general and pediatric rheumatology, rare diseases, patient advocacy, and other healthcare professionals. This article highlights the key […]
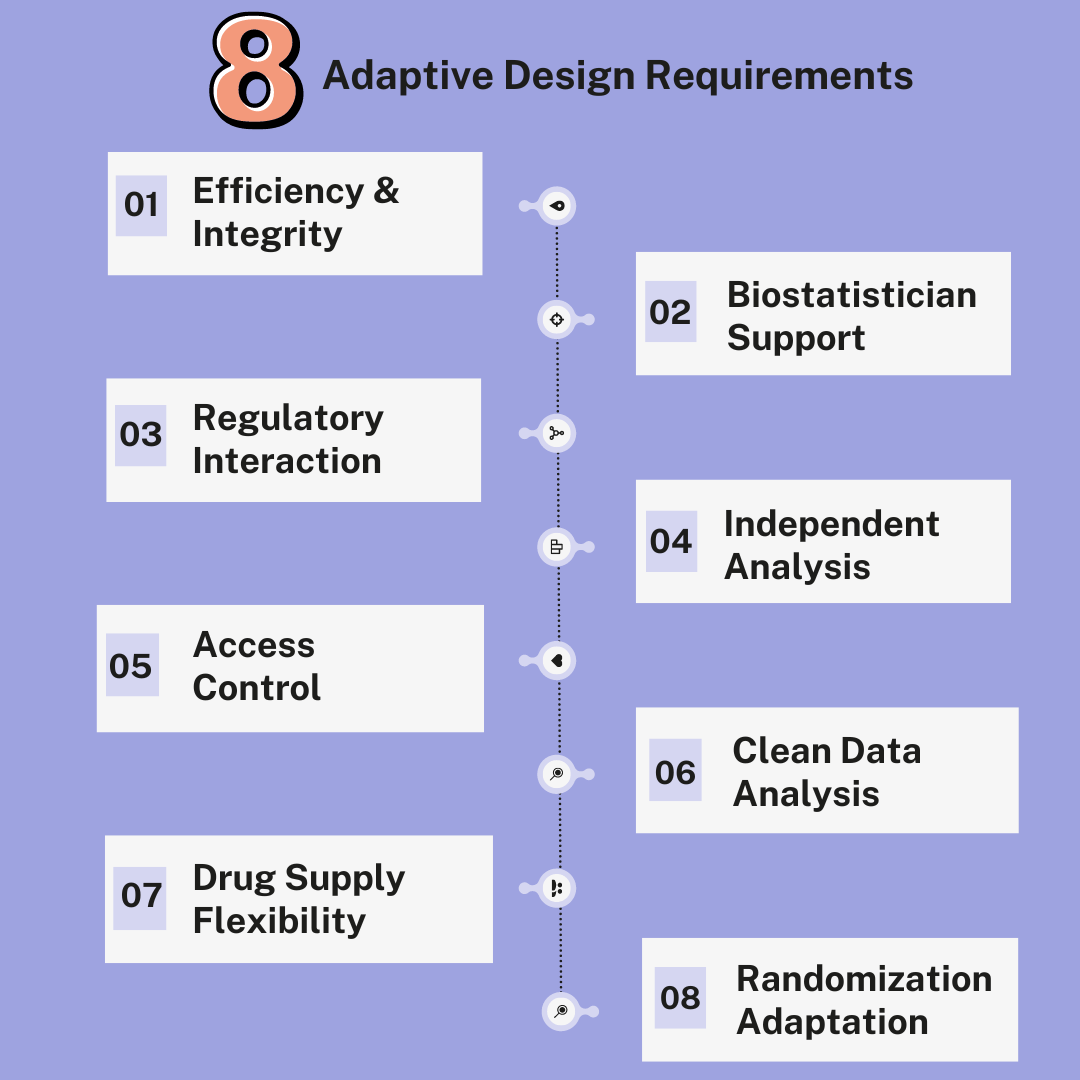
Adaptive design in clinical trials is a dynamic and innovative approach, but it requires certain key elements to ensure its successful implementation. Efficiency and Integrity The purpose of an adaptive trial design is to increase efficiency without compromising the integrity and validity of the results. For this to be accomplished, extensive and adequately maintained clinical […]

Did you know? Adaptive design offers numerous benefits that streamline the drug-development process and bring life-changing medications to patients more rapidly. Adaptive Study Design Help With: Reducing the costs of drug development Contributing to reducing the length of the trial Reducing the time to bring a drug to the market Allowing patients to access to […]
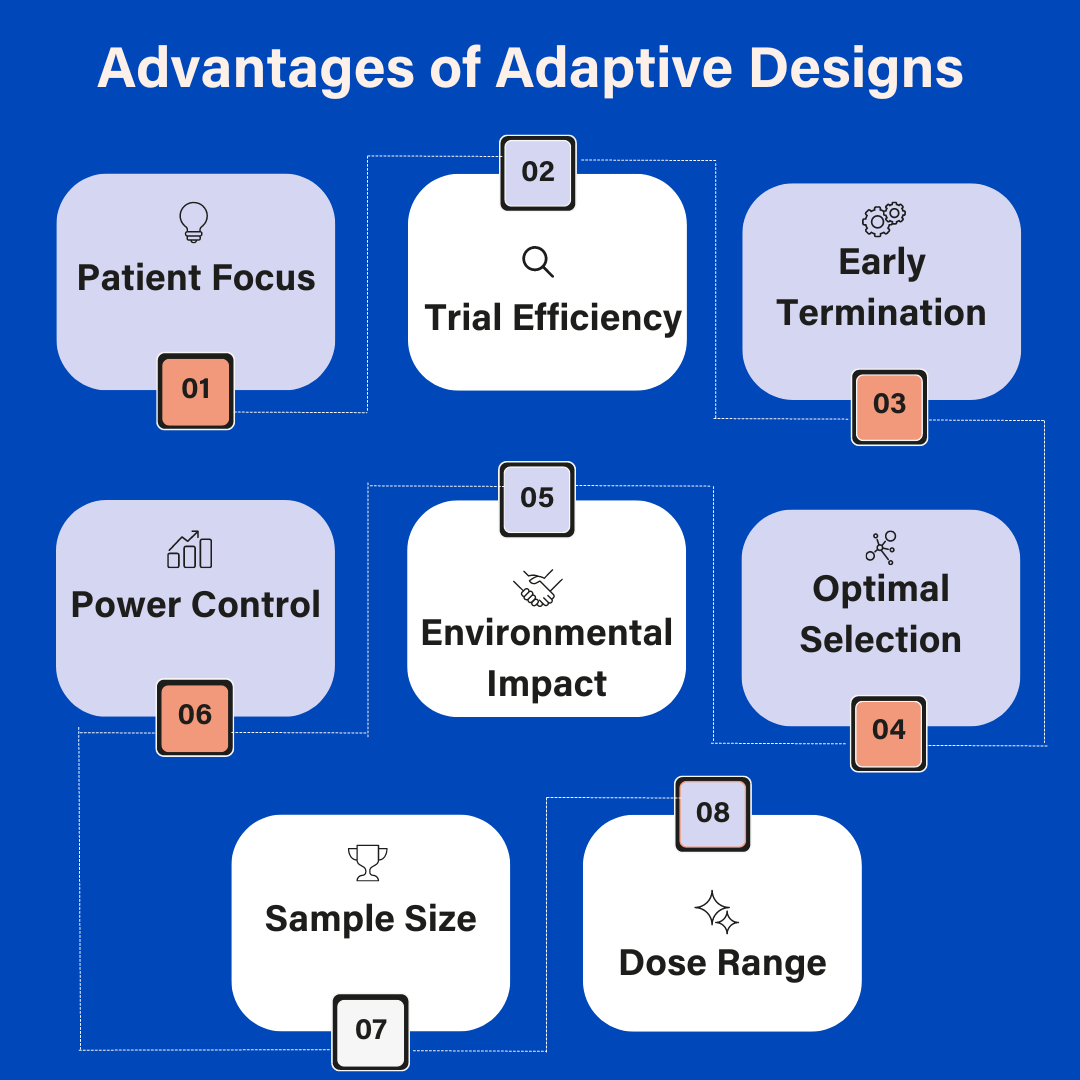
Explore our latest infographic to get a comprehensive overview of the advantages of adaptive design. Patient Focus Limiting patient exposure to drugs or doses that are not sufficiently effective. Trial Efficiency Allowing trial completion with fewer patients, enriching the trial population in a subgroup of patients for whom the medication is effective. Early Termination […]
Adaptive design is paving the way for more efficient and flexible studies. We are thrilled to present 8 types of adaptive designs that are shaping this transformation. Group sequential Allows for one of more pre-planned interim analyses and incorporates predetermined criteria for adaptations. Adaptation to the sample size Allows pre-planned adjustments to the sample […]
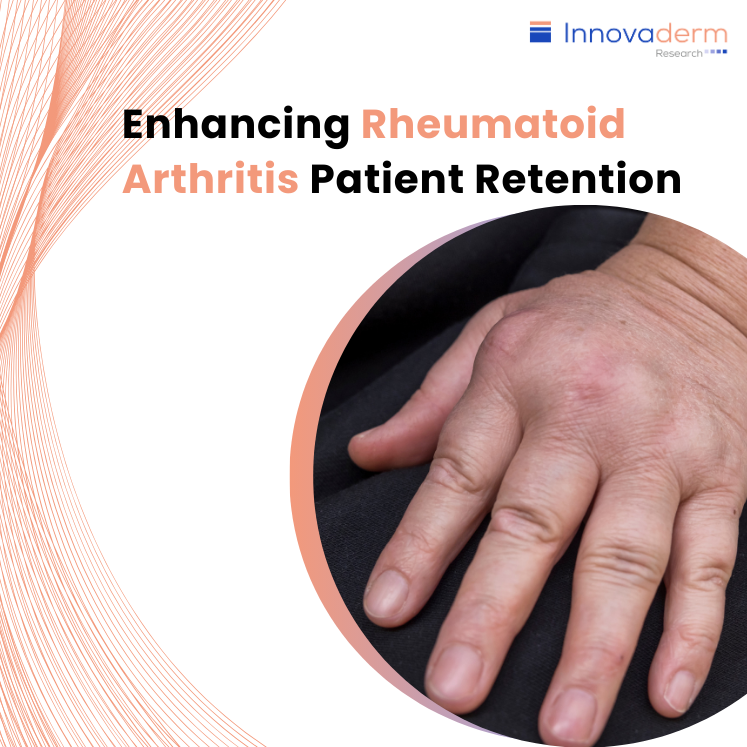
Table of contents: The Role of Quality Sites Retention Strategies Study Design Long-Term Safety Data and Brand Recognition Conclusion Maintaining patient participation is a critical factor that can determine the success or failure of a study, particularly in the context of rheumatoid arthritis (RA) clinical trials. Excellent patient retention is a hallmark of quality […]

Table of contents: The Rising Tide of Rheumatoid Arthritis and the Unmet Need Understanding the Patient Demographics and Comorbidities Patient Recruitment in RA Clinical Trials The Impact of Quality Sites on Recruitment The Rising Tide of Rheumatoid Arthritis and the Unmet Need Rheumatoid Arthritis (RA) is a severe autoimmune disease with profound physical, emotional, and […]
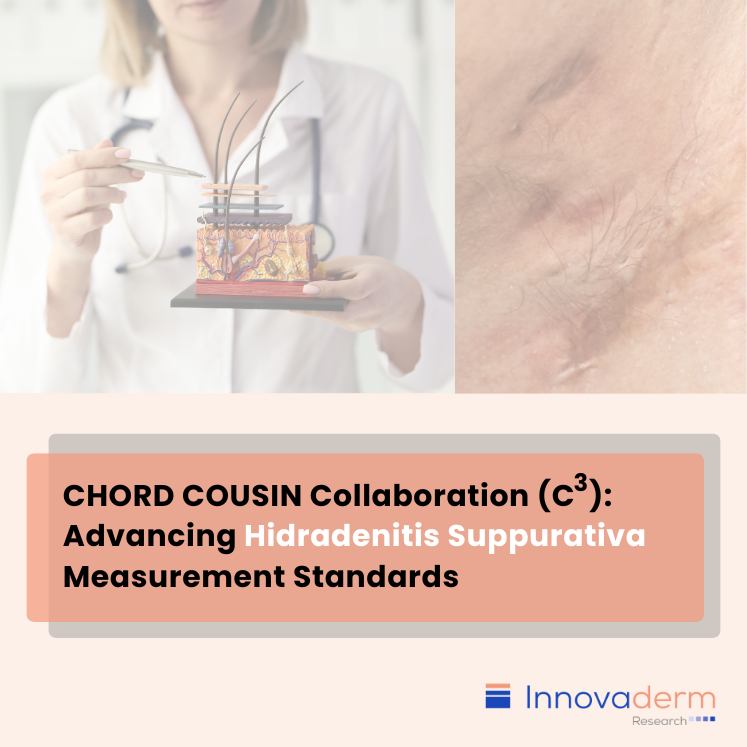
Table of contents: Introduction C3 HiSTORIC: Shaping the Future of HS Assessment It is essential to acknowledge some of the challenges faced by investigators when evaluating disease severity in hidradenitis suppurativa (HS) trials using the currently available outcome measures. These challenges extend to rare and uncommon diseases, where the absence of robust, rigorously developed, and […]

Table of contents: Current Endpoint in HS Trials: HiSCR Candidate Endpoints in HS Trials: IHS4 Candidate Endpoint in HS Trials: HS-IGA Characteristics of Candidate Endpoints The challenge of assessing hidradenitis suppurativa (HS), primarily resides with the raters rather than the measurement instruments. In an attempt to find potential solutions to high inter-rater variability in disease […]

Table of contents: The Role of the Disease The Role of the Measurement Metric The Role of the Raters When addressing the biggest hurdle in drug development for Hidradenitis Suppurativa (HS), the issue of high placebo response rates in clinical trials is unmistakably significant. The challenge lies in distinguishing between the mechanism of action (MOA) […]

Table of contents: Heterogeneous Morphological Presentation of HS Inter-rater Lesion and Measure Agreement and Reliability Among HS Experts As we explore the high response rate and the minimal difference between the active and placebo arms in hidradenitis suppurativa (HS), the question arises: “Could the high placebo response rate be more related to the rater’s use […]

Table of Contents What is considered as a reasonable placebo-response rate? Disease Severity Experience Monitoring Photographs Welcome to the second installment of the series on placebo response rate in atopic dermatitis (AD). This series aims to explore the complexities and nuances of placebo response rates in AD clinical trials and dive into the analysis […]

Table of contents Proof-of-Concept (POC) Studies How to Handle High Placebo-Response Rates Welcome to the final installment of the series on placebo response rate in atopic dermatitis (AD). The first articles of this series shed light on the complexities and nuances of placebo response rates in AD and on strategies on how to ensure a […]

Table of contents Factors Influencing Placebo-Responder Rate in AD Additional Factors Influencing Placebo-Responder Rate in AD Welcome to the first article in the series on placebo response rate in atopic dermatitis. This series aim to explore strategies on how to ensure a reasonable response rate and dive into the analysis of high response rates in […]

Table of contents Understanding Estimands Application of Estimands in Different Phases of Clinical Research Specifying Estimands for Secondary and Exploratory Endpoints Shared Cross-Functional Duties—Should estimand be only defined by biostatisticians? Benefits of Preliminary Conversation on Estimands Main Impacts of Statistical Analysis In essence, estimands are not a novel concept to clinical trials. The facets they […]

Our remarkable team has received high praise from yet another satisfied customer! "Working with Innovaderm for our alopecia areata trial has been a smooth and exceptional experience, marked by their extensive expertise, proactive approach, and seamless collaboration." Duong Nguyen, Head of Clinical Operations at Inmagene Biopharmaceuticals. “Innovaderm’s profound understanding of alopecia areata and their forward-thinking […]

Table of contents Alopecia Areata: The Condition The Severity of Alopecia Tool (SALT) Participation of Severe AA Patients in Clinical Trials Challenges in Limiting Studies to Severe AA Patients Implementing a Cap for Balanced Trials AA Treatment Alopecia Areata: The Condition Alopecia Areata (AA) is a medical condition characterized by hair loss that occurs […]
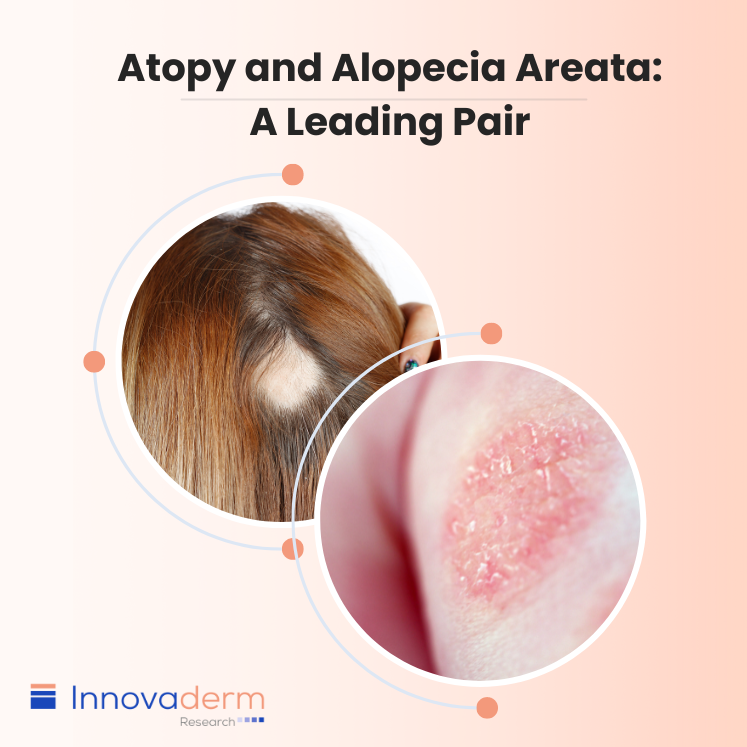
Table of contents Importance of Understanding Atopy as a Comorbidity in AA Relationship Between AD and AA Overview of Allergies in AA patients Significance of Eosinophils and Mast Cells Dupilumab as a Double Treatment of AA and AD Alopecia areata (AA) and atopy, which is a predisposition to develop allergic reactions, share a significant connection. […]

Table of contents Issues with Existing Treatments Alternative Options Ongoing phase 2 clinical trials Alopecia Areata (AA) is a condition characterized by unpredictable hair loss. The precise trigger of this immune response remains elusive, although it is thought to stem from an interplay of genetic and environmental factors. The indication has seen significant advancement in […]

Table of contents World’s largest growing market High volume European clinical trials Diverse geographical sites and patient populations Comprehensive regulatory agency Regulatory harmonisation of European clinical trials The European Union (EU) is listed as one of the most desirable regions to conduct a clinical trial and Innovaderm has positioned itself as a pacesetter in […]

Did you know that only a few dermatology studies employed adaptive study design in 2023? In the rapidly evolving landscape of clinical research, understanding the nuances of adaptive designs is crucial. From maintaining data blinding to navigating the complexities of interim analysis, there is a lot to unpack. Dive into the world of adaptive study […]

The success of any clinical study is dependent upon a sturdy protocol; the overall quality of the study depends on this very document. This is especially true for multicenter studies which involve many investigators and clinical staff. This article will explore the perspective of an investigator at Innovaderm Research, a Contract Research Organization (CRO) specialized […]

Innovaderm is excited to share a glowing testimonial from Ventyx Biosciences! "We would like to express our gratitude for the exceptional partnership we’ve had with Innovaderm. Our collaboration on a phase 2 study for moderate to severe plaque psoriasis was nothing short of remarkable. The expertise and motivation Innovaderm brought to the table were pivotal […]

Innovaderm is proud of its partnership with BioMimetix JV, LLC. Read what their Clinical Operation Director has to share on our expertise: “Our collaboration with Innovaderm has been truly outstanding. We initially sought their expertise in an atopic dermatitis trial management and right from the start, the team impressed us with their proficiency and professionalism. […]

Table of contents Cutaneous Dysbiosis Interplay S. aureus: Its Interaction and Impact on Skin S. aureus and Inflammation Focusing on the microbiome, Staphylococcus aureus (S. aureus) is a widespread bacterium that produces a multilayer biofilm, was shown to colonize the skin and play a critical role in Atopic Dermatitis (AD). Over 70% of people are […]
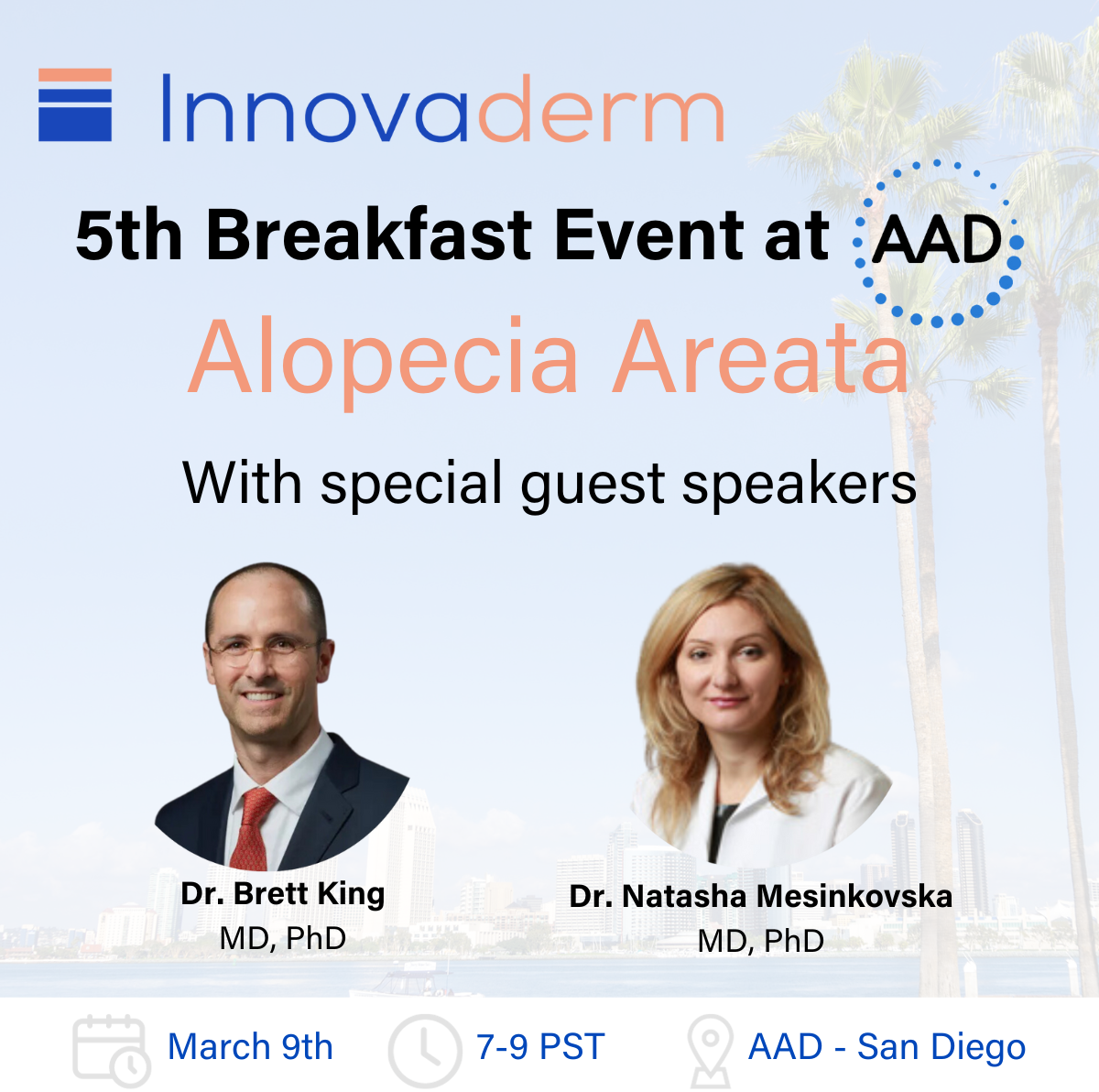
Innovaderm was in San Diego for the 2024 edition of the AAD annual meeting and hosted its 5th Breakfast Event: Exploring the Innovation and Research on Alopecia Areata (AA). Listen to our world-renowned AA key opinion leaders' presentations to learn about the disease and the latest research findings: Dr. Brett King - Clinical Evaluations and Current Treatments […]

Did you know that protocol amendments are not only common, but also very costly? In fact, as per a recent article by Getz K. et al., 57% of protocols had at least one substantial amendment with an average of 2.3 global amendments for phase 2 and phase 3 studies. The cost to implement one protocol amendment was $141,000 US for a phase 2 study and $535,000 US for a phase 3 study. To avoid unnecessary hassle and expenses, take the time to ensure your protocols are well written and top-notch quality.

The success of any clinical study is dependent upon a sturdy protocol; the overall quality of the study depends on this very document. This is especially true for multicenter studies which involve many investigators and clinical staff. This article will explore the best tips to deliver first-rate protocols in psoriasis to set you apart from the competition. Innovaderm Research Inc specializes in a spectrum of dermatological indications, namely acne vulgaris, atopic dermatitis, and psoriasis.

Dr. Robert Bissonnette from Innovaderm Research and Dr. Kim Papp from Probity Medical Research (PMR) met at the 2023 EADV for an engaging conversation focused on the intricacies of early phase studies and explored the significant challenges of conducting vitiligo studies. In this article, we shift the focus on alopecia areata (AA) studies insights, exploring the experiences of these seasoned dermatologists.

Amidst the bustling 2023 EADV in Berlin, Dr. Bissonnette from Innovaderm Research and Dr. Papp from Probity Medical Research (PMR) found a unique opportunity for an engaging conversation. The pair covered some of the main challenges encountered with conducting vitiligo studies and insights on alopecia areata (AA) studies. This article will focus on early phase studies.

Dr. Bissonnette from Innovaderm Research and Dr. Papp from Probity Medical Research (PMR) took a moment between presentations at the 2023 EADV for an interesting industry talk where the duo covered the intricacies of early phase studies and some of the main challenges encountered with conducting alopecia areata (AA) studies. However, the spotlight of this article will be on vitiligo studies. This piece aims to explore the complexities and the latest advancements in vitiligo research.

For the occasion of the 2023 EADV, Dr. Bissonnette from Innovaderm Research and Dr. Papp from Probity Medical Research (PMR) seized a rare moment to engage in a compelling conversation. These industry leaders share a common journey—the conception and evolution of their clinical research companies, which began as local endeavors in Canada and blossomed into influential international entities. The pair covered some of the main challenges of conducting vitiligo studies, the intricacies of early phase studies, and offered insights into alopecia areata (AA) studies. This exclusive interview will bring to light the pivotal role these entities play in shaping the future of clinical trials.

Innovaderm is thrilled to announce that our team was present at the AID Dermatology Summit in San Francisco on January 7th! Our very own Chief Medical Officer, Dr. Jasmina Jankicevic, delivered an insightful presentation titled “Clinical Development Strategy that Patients Need and Investors Want”. ⭐ Dr. Jankicevic's talk was nothing short of incredible, shedding light […]

Innovaderm is proud of its partnership with Nimbus Therapeutics for an investigational oral TYK2 inhibitor, in a Phase 2b trial for patients with moderate-to-severe psoriasis. Innovaderm’s reputed teams once again proved their expertise throughout the whole project, from the study design all the way to the trial reports, under tight timelines. “There are many CRO […]

Amidst the vibrant atmosphere of the EADV in Berlin, Dr. Bissonnette from Innovaderm Research and Dr. Papp from Probity Medical Research seized a rare moment between presentations to engage in a compelling conversation. These industry leaders share a common journey—the conception and evolution of their clinical research companies, which began as local endeavors in Canada […]

[MONTREAL, QUEBEC — December 19, 2023] Allergy recently published RAPT Therapeutics’ phase 1a/1b atopic dermatitis (AD) study, in which Innovaderm Research was a key partner, serving as the contract research organization (CRO). This was a first-in-human and proof of concept randomized, placebo-controlled Phase 1a/1b monotherapy study to evaluate the safety, tolerability, pharmacokinetics, pharmacodynamics, and CCR4 […]

Our skin is home to millions of microorganisms which play an important role in the innate and adaptive cutaneous immune system and the overall maintenance of our skin barrier. Dysbiosis has been shown to be associated with many skin diseases. Having a better understanding of the skin microbiome to allow dysbiosis reversal is critical for the development of new skin disease therapies. Integrating skin microbiome sample collection in clinical trials is a great opportunity to assess the impact of changes in microflora on skin disease state. Skin microbiome sampling can be a simple and quick process, however the following key elements must be considered to ensure quality samples:

Conventional avenues to collect samples for the analysis of biomarkers have proven to necessitate intrusive procedures, engendering patient aversion and potential procedural hazards. Several recent non-invasive sampling approaches such as tape stripping, transdermal patch analysis and microneedling are making their way in dermatology clinical trials. This article will focus on transdermal patch analysis in an attempt to bring emphasis on the patient’s well-being and the preservation of the fidelity of acquired data. Transdermal analysis patches (TAPS) noninvasively measure soluble proteins in the stratum corne.

Clinical biomarkers ushered in a new era of possibilities in medicine by profoundly impacting the way we investigate and develop new treatment options. Non-invasive techniques such as tape stripping, transdermal analysis patch, and microneedling are transforming the dermatology clnical landscape. Biomarkers in clinical trials measure changes in protein, gene expression, or other biochemical pathways associated with a specific condition or response to a drug.

There are several approaches to study biomarkers using non-invasive techniques such as tape stripping, transdermal analysis patch and microneedling . This article will focus on the tape stripping method.

Watch this exciting webinar presented by Innovaderm Research in collaboration with Altasciences and be part of: Deep Dive in Study Design Discussion on SAD-MAD-POC Best Practices and Innovations From our early phase experts: Dr. Ana Palijan, Associate Director, Early Phase Department, Innovaderm Research Dr. Beatrice Setnik, Chief Scientific Officer, Altasciences Fareheen Chowdhury, Project Manager, Early […]

The medical aesthetic market presents itself as a dynamic frontier with immense possibilities. Site selection, patient enrollment optimization, proper collection of relevant data, and proper operationalization set up, all have a direct influence on the progress of a medical aesthetic trial. Thus, closely exploring these key components will maximize a trial’s success.

The successful management of a clinical trial is a fine balance between multiple significant elements; rather it is the feasibility aspect, patient recruitment, optimized protocol, etc. The relationship with the clinical research site is often a point that goes unnoticed. Clinical research sites are integral to the success of clinical trials and the pathway to this success begins with maintaining positive professional rapports between sites and CROs. Fostering this kinship will be paramount to the overall flow of your trial as well as for future site collaborations, expediency and efficiency of the study start-up, facilitated budget and contract negotiations and, overall streamed line activation process.

The medical aesthetics industry is an increasingly lucrative and rapidly developing sector of healthcare, currently valued at $13.9 billion and expected to reach a total of $23.4 billion by 2027. Despite this enticing profit potential, developers must consider the numerous challenges and emerging trends that will shape the future of this market.

Dermal fillers, also known as injectable implants, soft tissue fillers, or wrinkle fillers have been on the medical aesthetic market for over 30 years and have a variety of specific technological characteristics and indications. The successful conduct of a medical aesthetic clinical trial is dependent upon the development of an optimal study protocol, meticulous planning, and diligent implementation. The key protocol considerations for development of dermal fillers are detailed below.

As the medical aesthetic market experiences robust growth, its accompanying technologies continue to evolve. Aesthetic trials use validated scales to evaluate the severity and improvement of a given condition in order to support efficacy endpoints. These evaluations are often performed as live local assessments at the site. However, as objective as these scales aim to be, these methods of assessment often lack standardization and introduce biases that can affect the reliability of the final results.

Innovaderm was in New Orleans for the 2023 edition of the AAD congress and hosted its 4th Breakfast Event: Spotlight on Hidradenitis Suppurativa (HS). Listen to our world-renowned dermatology key opinion leaders' presentations to learn about HS and the latest research findings: Dr. Amit Garg, MD - Challenges of Current Scales & Development of Novel […]

Click here for a PDF version of this case study Outcomes Innovaderm successfully transitioned this rescue study from another CRO and this resulted in: More than doubled patient enrollment in 1 month Started enrollment 1 month faster than initial projection All 5 newly added sites were actively recruiting Study Characteristics Study Phase: IIa Study […]

Tape Stripping is a non-invasive option for the study of biomarkers in dermatology studies. Innovaderm is sharing its 3 best tips when performing tape strip collections. Let’s cover our bases, what is tape stripping? Tape stripping is used in dermatology studies to sample skin surface material by collecting a thin layer of cells from the […]

Innovaderm's Associate Director - Risk Based Quality Management, Rohit Gupta, contributed to PHUSE's white paper entitled: “Centralized Monitoring: Exploring the Considerations and Challenges of Implementation”. Innovaderm is proud to have been involved in the development of such a document addressing: 1. The key success measures & best practices to ensure a well-connected end-to-end component of […]

April is dedicated to raising awareness around Rosacea. One of Innovaderm’s all-star project managers, Nalanie Johnson, shared some tips that are crucial when managing a clinical trial to the complexity of a therapeutic indication such as Rosacea: 1. In managing Rosacea studies, it is important to understand the indication and patient flow. Most patients will […]

Click here for a PDF version of this case study Outcomes Innovaderm successfully managed this HS study and this resulted in: Enrollment completed 3 months ahead of schedule Study Characteristics Study Phase: II Patient Population: Hidradenitis Suppurativa IP Route of Administration: Systemic - Subcutaneous Site distribution: 25 in North America and 16 in Europe […]

📣Attention all clinical trial sponsors who are planning to conduct a study in the EU or EEA! Beginning on January 31, 2023, the Clinical Trials Information System (CTIS) must be used to submit any new clinical trial application in these regions. This new process permits a single application to secure authorizations in up to 30 […]

Click here for a PDF version of this case study Outcomes Innovaderm successfully managed this PPP study and this resulted in: Enrollment completed 3 weeks ahead of schedule; Successful study and satisfied sponsor Study Characteristics Study Phase: II Patient Population: Palmoplantar pustulosis IP Route of Administration: Systemic Sites Distribution: 17 sites in 2 countries […]

Watch this exciting and informative webinar about best practices on medical aesthetic studies from our industry experts. You will learn about: Insight into industry trends and key study design considerations; How to execute studies quickly without compromising data quality; Benefits and limits of qualitative image-derived evaluations From our industry Experts: Jasmina Jankicevic, Chief Medical Officer, […]

Click here for a PDF version of this case study Study Characteristics Study Phase: II Patient Population: 126 patients with Notalgia Paresthetica (NP) (Pruritus associated with NP) IP Route of Administration: Systemic (oral) Site Distribution: 33 sites (USA and Canada) Study Procedures: Photographs, Pharmacokinetics, Electrocardiograms Study Challenges Patient awareness: Challenge in advertisement as […]

Please join us for this unique webinar to learn more about non-invasive methods to collect and evaluate skin biomarkers in clinical trials. You will learn more about: Tape stripping techniques Sensitivity of various cutaneous biomarkers Non-invasive sample collection methods for skin protein and RNA analysis Questions? Contact us Newsletter Join our science community […]

Click here for a PDF version of this case study Outcomes Innovaderm’s outstanding support on this alopecia areata study resulted in a rapid increase in enrollment rates and the successful management of this trial Study Characteristics Study Phase: II Patient Population: Alopecia Areata IP Route of Administration: Systemic Sites distribution: 12 sites (CAD-USA) Complex […]

Click here for a PDF version of this case study Outcomes Innovaderm successfully managed this AD study and this resulted in: Enrollment completed 2 months earlier than planned; Innovaderm CRU recruited 33% of patients Study Characteristics Study Phase: Ib Patient Population: 36 subjects with moderate to severe AD IP Route of Administration: Systemic - […]

Click here for a PDF version of this case study Outcomes Innovaderm successfully managed this acne study and this resulted in: Enrollment completed 1 month earlier than planned Study Characteristics Study Phase: II Patient Population: Acne vulgaris (enrolling pediatrics & adults) IP Route of Administration: Systemic Sites distribution: 15 sites (USA) Study […]

Click here for a PDF version of this case study Outcomes Innovaderm successfully transitioned this rescue study from another CRO and this resulted in: Increase in enrollment of over 10x Tripled site performance post transition On-time patient recruitment Study Characteristics Study Phase: II Study Status: Rescue study Patient Population: Palmoplantar pustulosis (PPP) IP Route […]

Don't miss this opportunity to join us for an informative session on skin barrier dysfunction where you will learn about: The disorder and its role in atopic dermatitis The impact of immune dysregulations and microbiome abnormalities on inherent skin barrier anomalies Methods to study skin barrier integrity in clinical trials Questions? Contact us Newsletter […]

In recent years, therapeutics for dermatologic conditions have become a subspecialty of growing interest in the Clinical Research Organisation (CRO) industry, with clinical research in the area focused on a wide range of skin indications, such as acne vulgaris, psoriasis, and atopic dermatitis. What is atopic dermatitis? Atopic dermatitis (AD), also known as atopic eczema, […]

In recent years, therapeutics for dermatologic conditions have become a subspecialty of growing interest in the Clinical Research Organisation (CRO) industry, with clinical research in the area focused on a wide range of skin indications, such as acne vulgaris, psoriasis, and atopic dermatitis. What is atopic dermatitis? Atopic dermatitis (AD), also known as atopic eczema, […]

n recent years, dermatologic therapeutics has become a subspecialty of growing interest in the CRO industry, with clinical research in the area focused on a wide range of skin indications such as acne vulgaris, psoriasis, and atopic dermatitis.

Watch this exciting and informative webinar on how to write the best protocols in dermatology. You will learn about: protocol development and associated tools; protocol considerations for key dermatology indications; clinical assessments, eligibility criteria, study design and more! Questions? Contact us Newsletter Join our science community with Press Forward, our quarterly newsletter. Get […]

Watch this unique webinar to learn more about how to handle missing data in dermatology trials. You will learn more about: How missing data arises in dermatology studies Which statistical methods to choose to deal with missing data Which methods may be preferred by the Regulatory Bodies Questions? Contact us Newsletter Join our science […]